Electron configuration of Yttrium Y Kr 4d 1 5s 2. The electron arrangement of an atom at its lowest possible energy state is known as the ground state electron configuration.

Give The Ground State Electron Configuration For Rb A Clutch Prep

Orbitals Periodic Table
1
The electronic configuration is given by ns 1.
Rubidium electron configuration. Electron affinity is the amount of energy change ΔE that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Concise Form of Electron Configuration Notation. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass.
Ruthenium Kr 4d 7 5s 1. Periodic table Strontium Electron configuration. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2.
The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers electron configurations and chemical properties. Electron Configuration Chart for All Elements in the Periodic Table. Molybdän Kr 4d 5 5s 1.
Nevertheless check the complete configuration and other interesting facts about Rubidium that most people dont know. Yttrium Kr 4d 1 5s 2. Electron configuration of Molybdenum is Kr 4d5 5s1.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. In the case of Rubidium the abbreviated electron configuration is Kr 5s1. Hydrogen H 1s 2.
Sodium a øan a as ap 4. Electron Configuration and Oxidation States of Molybdenum. Full electron configuration of strontium.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 1. 2270 Atomic Number. Electron Configurations Worksheet - Answers Write the complete ground state electron configurations for the following.
They tend to lose the outer shell electron to form cations with charge 1 monovalent ions. Each element has a unique atomic structure that is influenced by its electronic configuration which is the distribution of electrons across different orbitals of an atom. Concise Form of Electron Configuration Notation Element.
Write the full electron configuration noble gas electron configuration and fill in the orbital diagrams for the following elements. Where are the Electrons. Rubidium cannot be stored under atmospheric oxygen as a highly exothermic reaction will ensue sometimes even resulting in the.
Possible oxidation states are 6. Using the electron configuration and principles of physics chemists can predict an atoms properties such as stability boiling point and conductivity according to the Los Alamos National. Electron shell 1 has the lowest energy and its s-orbital is the first to be filled.
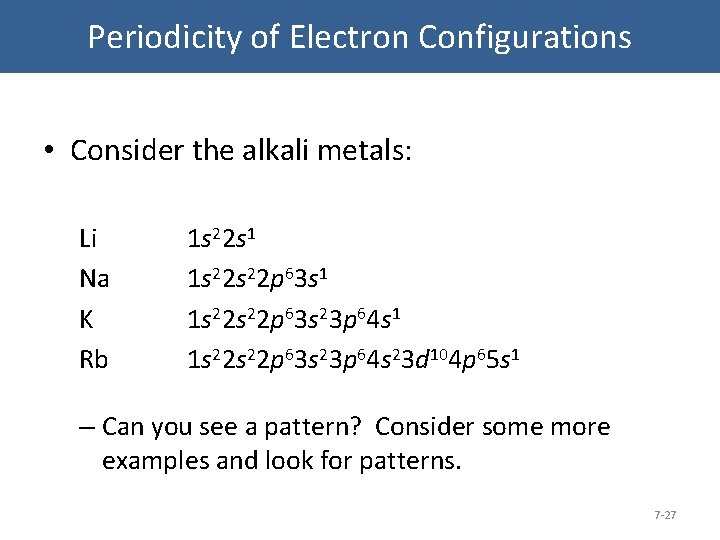
Rubidium is a very soft silvery-white metal in the alkali metal group. The electron configuration shows the distribution of electrons into subshells. Alkali metals have one electron in their valence shell.
Rubidium-85 is the dominant form accounting for 72 per cent of the total while most of the remainder is the radioactive rubidium-87 which has a half-life of 50 billion years. Rubidium Kr 5s 1. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
Nitro en 3s B 2. Electron configuration of Strontium Sr Kr 5s 2. Periodic Table of the Elements Electron configuration of Strontium.
Rhodium Kr 4d 8 5s 1. Actinium Atomic Weight. There are 118 elements in the periodic table.
Chromium Orbiä Filhg 3ci. Niob Kr 4d 4 5s 1. 2 8 18 8 1.
Hydrogen H 1s 2. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. For example the electronic configuration of lithium is given by 1ns 1 2ns 1.
Nickel 6 Vanadium Is 7 Cop er. Strontium Kr 5s 2. In many cases multiple configurations are within a small range of energies and the irregularities shown below do not necessarily have a clear relation to chemical behaviour.
Rubidium metal shares similarities to potassium metal and caesium metal in physical appearance softness and conductivity. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Concise Form of Electron Configuration Notation Element.
Electron configuration of Rubidium Rb Kr 5s 1. Fluorine has the greatest electronegativity of all the elements and the heavier alkali metals such as potassium rubidium and cesium have the lowest electronegativities. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. Electronic Configuration of Alkali Metals. Rubidium is the chemical element with the symbol Rb and atomic number 37.
Technetium Kr 4d 6 5s 1. Note that these electron configurations are given for neutral atoms in the gas phase which are not the same as the electron configurations for the same atoms in chemical environments. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
Learn more about the definition of the ground state electron. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 5s 2. To save room the configurations are in noble gas shorthandThis means part of the electron configuration has been replaced with the element symbol of the noble gas symbol.
Zirconium Kr 4d 2 5s 2. Lithium oxygen calcium titanium rubidium lead erbium 1s22sl Is 2s 2P 6262 Is 2s 2P 3s 3p 4s 6262 Is 2s 2P 3s 3p 4s 3d2 6262 10 6 1 Is 2s 2P 3s. In other words when the electron is added to a neutral atom the energy is either released or absorbed.
Palladium Kr 4d 10. Concise Form of Electron Configuration Notation. Click here to buy a book photographic periodic table poster card deck or 3D print based on the images you see here.
Transition Metals Electron Configuration. IiDeduce the full electron configuration of a rubidium atom. This list of electron configurations of elements contains all the elements in increasing order of atomic number.
Strontium complete electron configuration. Electron configuration of every element in the periodic table Element Electron configuration 1 Hydrogen 1s1 2 Helium 1s2 3 Lithium 1s22s1 4 Beryllium 1s22s2 5 Boron 1s22s22p1 6 Carbon 1s 22s22p 7 Nitrogen 1s 22s 2p3 8 Oxygen 1s22s22p4 9 Fluorine 1s22s22p5 10 Neon 1s22s22p6 11 Sodium 1s22s22p63s1 12 Magnesium 1s22s22p63s2 13 Aluminum 1s 22s 2p63s 3p1 14 Silicon. Neon Is 23 5.
1 Page 6 of 57 e A sample of rubidium contains the isotopes 85Rb and 87Rb only. The isotope 85Rb has an abundance 25 times greater than that of 87Rb Calculate the relative atomic mass. Rubidium strontium yttrium.
Ac Name of Element. 2 8 18 8 2. Rubidium Overview Rubidium Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 p6 5 s1 Abbreviated Electron Configuration Kr 5s1 Sources.
The radioactive isotope decays to form strontium-87.

Rb Electron Configuration Rubidium Ion Electron Configuration Electrons Configuration
Rubidium Atomic Structure Stock Image C018 3718 Science Photo Library

Group 1 Elements Alkali Metals Emedicalprep
Electron Configurations Of Main Group Elements Ck 12 Foundation

Explain V Electron Configuration Uths Demo Course
Electron Structure Of The Atom Electron Configuration 7

Alkali Metals

Chapter 11 Exercise Key


