Value is a guess based on periodic table trend. For example the electron configuration of the neon atom is 1s2 2s2 2p6 using the notation explained below.

Orbital Diagram For Vanadium V Vanadium Electron Configuration Periodic Table

More On Electron Configuration Generalchemistoncall
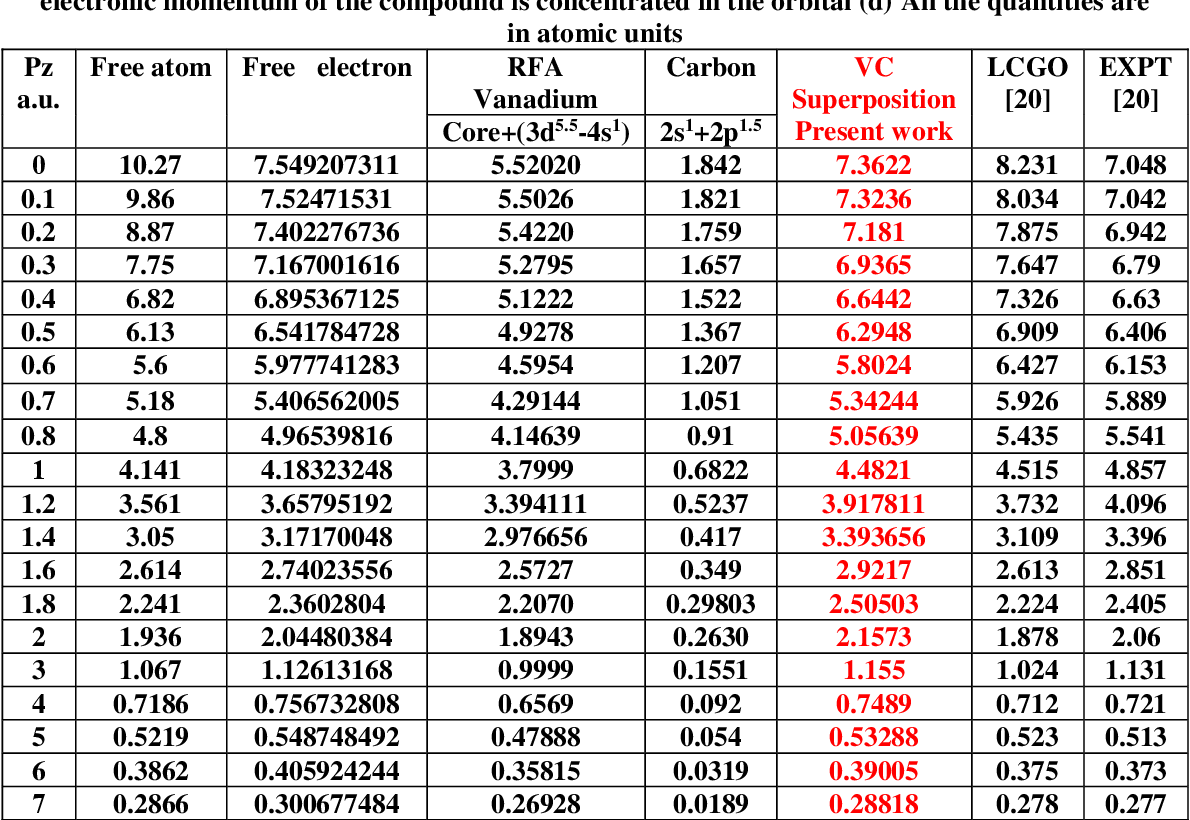
Electronic Configuration Of Vanadium Carbide Vc Semantic Scholar
The electron configuration shows the distribution of electrons into subshells.

Vanadium electron configuration. Neon Is 23 5. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers electron configurations and chemical properties. Enter the name or symbol.
This list of electron configurations of elements contains all the elements in increasing order of atomic number. Ar 4s 2 3d 3. 2 8 11 2.
Carbon Electron Configuration. Electron configuration was first conceived under the Bohr model of the atom and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons. Instead of 23 electrons to distribute in orbitals there are 5.
Chrom Ar 3d 5 4s 1. An electron shell is the set of allowed states that share the same principal quantum number n the number before the letter in the orbital label that electrons may occupy. Thought it was 1s2 2s2 sp2.
Ac Name of Element. Screening Constant and Z eff for Vanadium. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 3 4s 2.
Value is a guess based on periodic table trend. Andrés Manuel del Río discovered compounds of vanadium in 1801 in Mexico by. Where i is a number between 0 and 14.
The noble gas in the configuration is denoted E in brackets. 23 - 18 5. Mangan Ar 3d 5 4s 2.
If you guys have come across our recent article then it would be easy for you all to understand the conceptBut if you are new here and looking for the information related to the carbon element and its electronic configuration then today we will help you with some of the things and if you will be here till the last line surely you will go with some knowledge. Electron configuration of Chromium Cr Ar 3d 5 4s 1. Nickel 6 Vanadium Is 7 Cop er.
To find the valance electrons that follow subtract the atomic numbers. The first example occurs in the case of the lanthanoids elements having atomic numbers between 57 and 71The lanthanoids have the general electron configuration Kr4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. Electron configuration is very important to know where the electrons are located around the atoms.
Write the full electron configuration noble gas electron configuration and fill in the orbital diagrams for the following elements. 1s 2 2s 2 2p 6 3s 2 3p 6. The third major category of elements arises when the distinguishing electron occupies an f subshell.
Aluminum Al - Relative atomic mass A of aluminum is 27. There are 118 elements in the periodic table. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 3 4s 2 or Ar 3d 3 4s 2 But what happens next.
Number of electrons present in s p d and f orbitals can be. Kupfer Ar 3d 10 4s 1. What is the electronic configuration of carbide C-4.
It is a hard silvery-grey malleable transition metalThe elemental metal is rarely found in nature but once isolated artificially the formation of an oxide layer passivation somewhat stabilizes the free metal against further oxidation. VanadiumIV has one unpaired 3d electron that coupled with the nuclear spin is exquisitely diagnostic in EPR spectroscopy - the vanadyl ion VO 2 is a sensitive spectroscopic probe that has been used to elucidate enzyme active site structure as well as catalytic activity. Vanadium has atomic number 23 and the electron configuration according to the Slaters rules for shielding electrons 1s 2 2s 2p 8 3s 3p 8 3d 3 4s 2Therefore the screening constant for 4s electron 2 10.
Vanadium atoms have 23 electrons and the shell structure is 28112. Description Your user agent does not support the HTML5 Audio element. Germanium Ar 3d 10 4s 2 4p 2.
Learn more about properties density and melting point of aluminium along with atomic mass of aluminium. Vanadium Ar 3d 3 4s 2. Concise Form of Electron Configuration Notation Element.
Now there is enough information to write the electron configuration. N atomic physics and quantum chemistry the electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals. Actinium Atomic Weight.
Value is a guess based on periodic table trend. Value is a guess based on periodic table trend. Nickel Ar 3d 8 4s 2.
Gallium Ar 3d 10 4s 2 4p 1. Why is Electron configuration important. In the case of Vanadium the abbreviated electron configuration is Ar 3d3 4s2.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3. Sodium a øan a as ap 4. Electronic configuration is important in the following ways.
Each element has a unique atomic structure that is influenced by its electronic configuration which is the distribution of electrons across different orbitals of an atom. Ar4s23d3 - I know that this is Vanadium Xe6s24f1 - I thought that this was Lu but it counts it. Aluminum is a soft silvery-white ductile metal in the boron group.
The electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals. Vanadium is a chemical element with the symbol V and atomic number 23. Electron configuration chart of all Elements is mentioned in the table belowThe Shorthand electron configuration or Noble gas configuration.
Concise Form of Electron Configuration Notation. Electron Configuration Chart for All Elements in the Periodic Table. Hydrogen H 1s 2.
Electron configuration of Vanadium V Ar 3d 3 4s 2. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2 or Ar 3d 2 4s 2. Electron configuration of every element in the periodic table Element Electron configuration 1 Hydrogen 1s1 2 Helium 1s2 3 Lithium 1s22s1 4 Beryllium 1s22s2 5 Boron 1s22s22p1 6 Carbon 1s 22s22p 7 Nitrogen 1s 22s 2p3 8 Oxygen 1s22s22p4 9 Fluorine 1s22s22p5 10 Neon 1s22s22p6 11 Sodium 1s22s22p63s1 12 Magnesium 1s22s22p63s2 13 Aluminum 1s 22s 2p63s 3p1 14 Silicon.
Notes on the Electron Configuration of particular elements. To save room the configurations are in noble gas shorthandThis means part of the electron configuration has been replaced with the element symbol of the noble gas symbol. Therefore there is one more way that you can learn the.
Therefore many users will not be able to remember it and some will be those who will find it very difficult to write it. 2270 Atomic Number. Vanadium Z23 Using the filling sequence.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 3 Writing the electronic configuration. Vanadium Overview Vanadium Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d3 Abbreviated Electron Configuration Ar 3d3 4s2 Sources. Transition Metals Electron Configuration.
Nevertheless check the complete configuration and other interesting facts about Vanadium that most people dont know. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. Value is a guess based on periodic table trend.
Eisen Ar 3d 6 4s 2. Cobalt Ar 3d 7 4s 2. Which neutral atoms have the following electron configurations in either a ground state or excited state.
Zink Ar 3d 10 4s 2. Vanadium Electron Configuration of V will be written as. The ground state electronic configuration of neutral vanadium is Ar3d 34s 2 and the term symbol of vanadium is 4 F 32.
Thus in the building-up process for the lanthanoids. Writing the electronic configuration. Nitro en 3s B 2.

Question Video Identifying The Electronic Configuration Of The Vanadium V Ion Nagwa

Vanadium V Element 23 Of Periodic Table Elements Flashcards
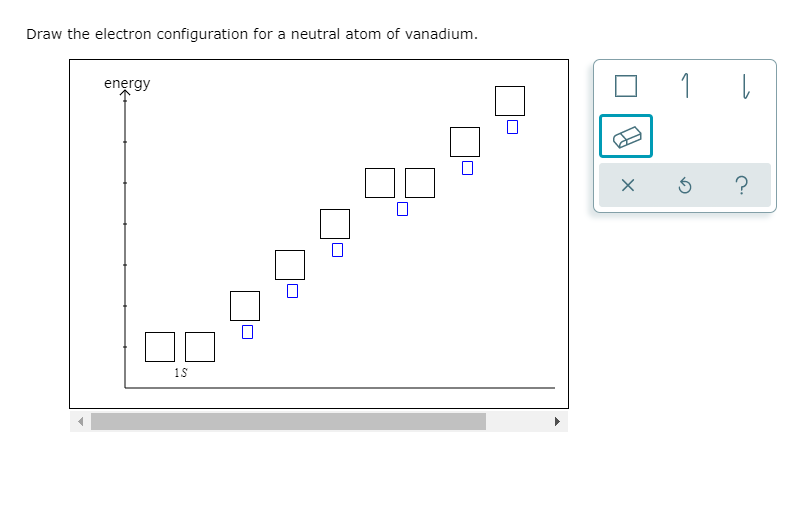
Solved Draw The Electron Configuration For A Neutral Atom Of Chegg Com
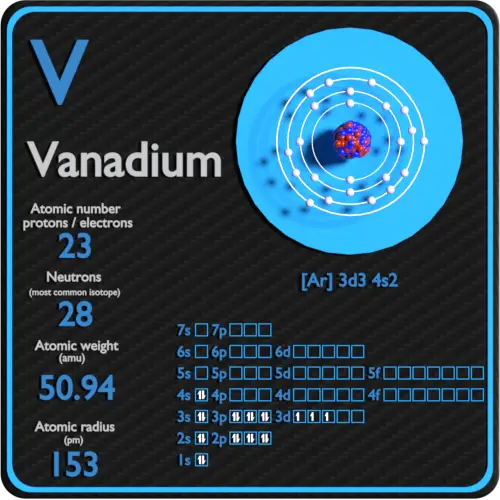
Vanadium Protons Neutrons Electrons Electron Configuration

Electron Orbital Diagram Of Vanadium Chemistry Stack Exchange
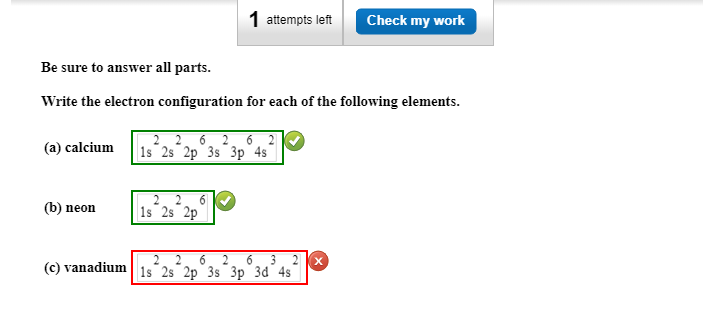
Solved Attempts Left Check My Work Be Sure To Answer All Chegg Com

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
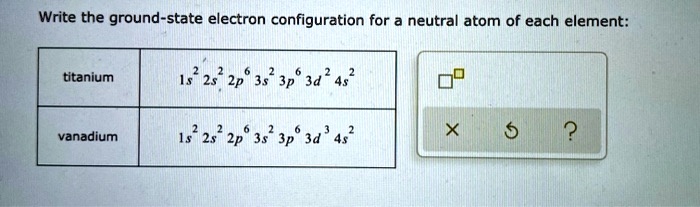
Solved Write The Ground State Electron Configuration For A Neutral Atom Of Each Element Titanium 1s 2s 2p 3s Jp 3d 4s Vanadium 2s2 2p6 3s Gp 3d